साधारण सेल


साधारणतया तरल अम्ल जस्तै सल्फ्युरिक अम्लमा तामा धातु र जस्ता धातुका पाताहरूलाई डुबाएर तयार पारिएको एक प्रकारको सेललाई साधारण सेल भनिन्छ । यसबाट थोरै मात्रामा विद्युत उत्पादन गर्न सकिन्छ ।
रसायान-विज्ञान
[सम्पादन गर्नुहोस्]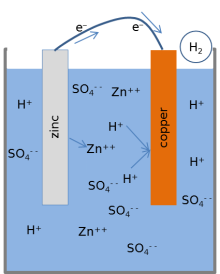
Most textbooks present the following model for the chemical reactions of a lemon battery.[१][२][३] When the cell is providing an electric current through an external circuit, the metallic zinc at the surface of the zinc electrode is dissolving into the solution. Zinc atoms dissolve into the liquid electrolyte as electrically charged ions (Zn2+), leaving 2 negatively charged electrons (e−) behind in the metal:
This reaction is called oxidation. While zinc is entering the electrolyte, two positively charged hydrogen ions (H+) from the electrolyte combine with two electrons at the copper electrode's surface and form an uncharged hydrogen molecule (H2):
This reaction is called reduction. The electrons used in the copper to form the molecules of hydrogen are transferred from the zinc through an external wire connecting between the copper and the zinc. The hydrogen molecules formed on the surface of the copper by the reduction reaction ultimately bubble away as hydrogen gas.

